SUPERCRITICAL FLUIDS
Supercritical fluids: a supercritical fluid is any substance at a temperature and pressure above its critical point. It can diffuse through solids like a gas and dissolve materials like a liquid. In addition, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "tuned". Supercritical fluids are suitable as substitutes for organic solvents in a wide range of industrial and laboratory processes. CO2 is one of the most widely used supercritical fluids worldwide.
What are the supercritical fluids used for?
Supercritical fluid graphs
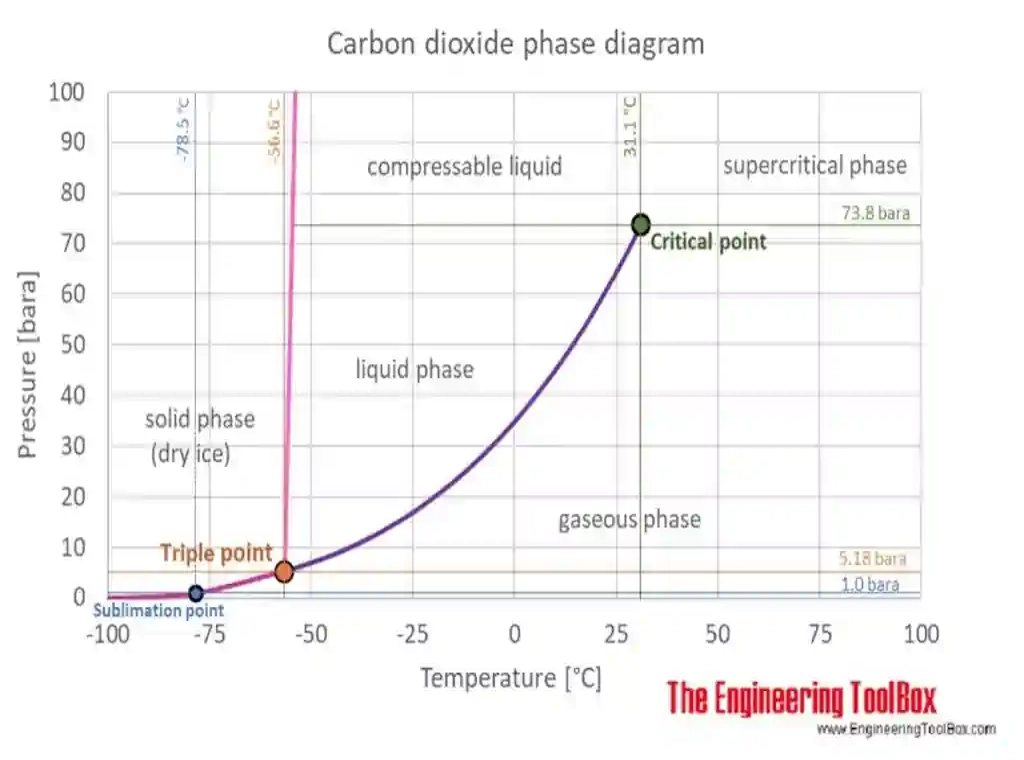
co2 phase diagram
Representation of triple and supercritical points
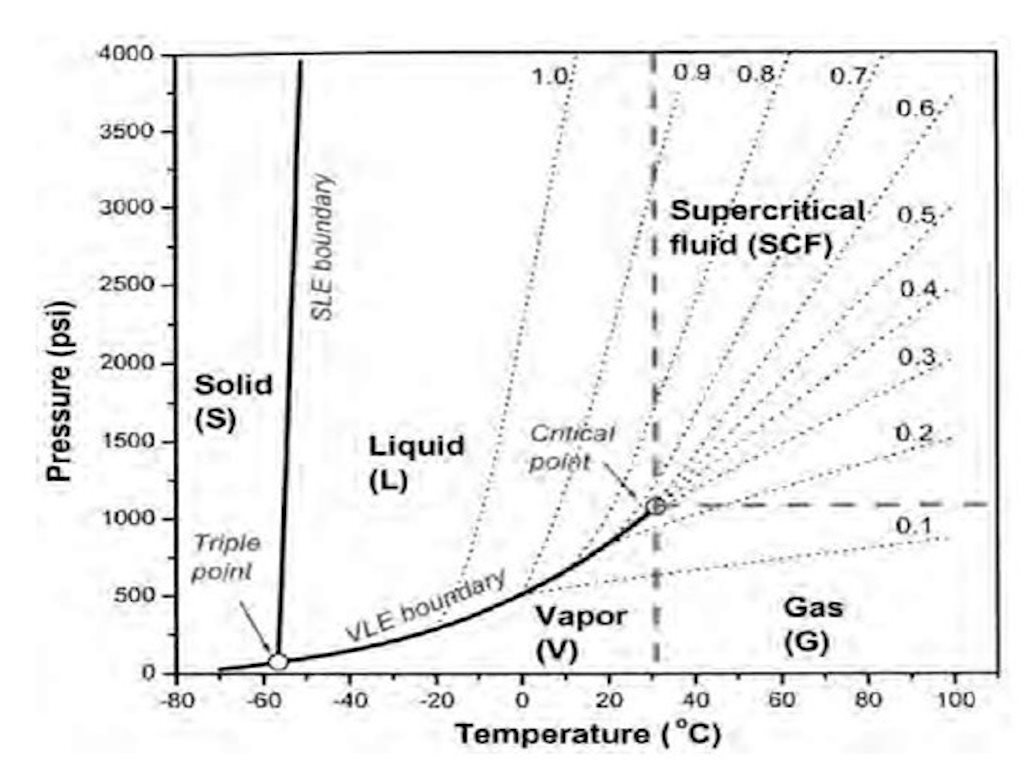
density diagram
Representation of density with varying pressure and temperature.
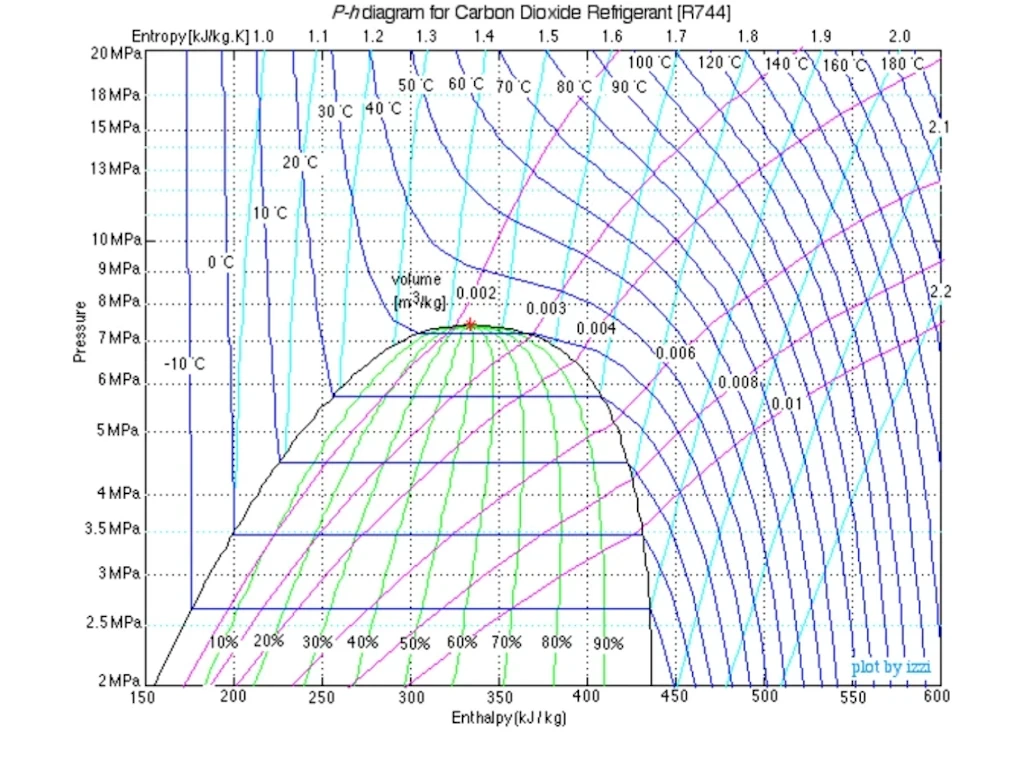
MOLLIER DIAGRAM
Representation of thermodynamic parameters.
ADVANTAGES OF SUPERCRITICAL FLUIDS: CO2
CO2 supercritical fluid, is the most common among supercritical fluids, it is very attractive for many reasons:
• very cheap and abundant in pure form (for food use) throughout the world;
• non-flammable and non-toxic;
• environmentally friendly, since it is a non-polluting gas and most of the CO2 is produced from waste streams;
• critical temperature at 31°C, allowing operations at temperatures close to ambient temperature,
• avoiding product alterations;
• critical pressure of 74 bar, which leads to an "acceptable" operating pressure, generally between 100 and 350 bar.
• very cheap and abundant in pure form (for food use) throughout the world;
• non-flammable and non-toxic;
• environmentally friendly, since it is a non-polluting gas and most of the CO2 is produced from waste streams;
• critical temperature at 31°C, allowing operations at temperatures close to ambient temperature,
• avoiding product alterations;
• critical pressure of 74 bar, which leads to an "acceptable" operating pressure, generally between 100 and 350 bar.
Cosolvent: solvent added to the main supercritical fluids (generally carbon dioxide) to modify their solvent power towards "polar" molecules since the fluid itself is capable of dissolving only "non-polar" molecules; Generally the cosolvent is chosen from short chain alcohols, esters or ketones. For obvious reasons, in many cases ethanol is preferred, since it is abundant and cheap in pure forms (for food use, for pharmacopoeia), little dangerous for the environment and little toxic (as is well known!). >
CO2 is available both in commercial cylinders or spheres of limited volume, as well as in bulk in tanks containing 4 to 30 tons of liquefied gas and not as a supercritical fluid at a pressure of 18 bar and a temperature around –18°C. These tanks work directly with cryogenic trucks. The price is very sensitive to the quantity delivered, since transportation is the main cost because carbon dioxide is available at a very low price in various chemical plants.
Since most SCF plants require liquid carbon dioxide at a pressure of around 45 bar, the liquefied gas must be compressed from the storage pressure (18 bar) to 50 bar using a pump. For applications in the food and pharmaceutical industry it is mandatory to use a "dry" pump to avoid any contamination by the pump lubricant..

Lorem Ipsum has been the industry's standard dummy text ever since the 1500s, when an unknown printer took a galley of type and scrambled it to make a type specimen book.



